
We can calculate the standard entropy change, S, for a reaction in the. Although it isn't trivial in general, you can check how the formula simplifies for processes mentioned below. A chemical reaction in which the number of moles of gas increases (the sum of. The general formula for work done by the gas is expressed as: ∫p(V)dV if we consider pressure as the function of volume. In real gases, these parameters differ from theoretical ones, but it's already contained in our thermodynamic processes calculator. It is commonly stated as the law of conservation of energy i.e., energy can neither be created nor be destroyed.

3 * R for gases with more complex molecules. The equation 6.1 i.e., U q+ w is mathematical statement of the first law of thermodynamics, which states that The energy of an isolated system is constant.If you have information about one or more reactants, select Reactant Amount Given Otherwise, select Product Amount Given. 3) The total energy of universe is always constant. Example: Cu + O2 + CO2 + H2O Cu2 (OH)2CO3. For a spontaneous process, the total entropy change, S total is always greater than zero.
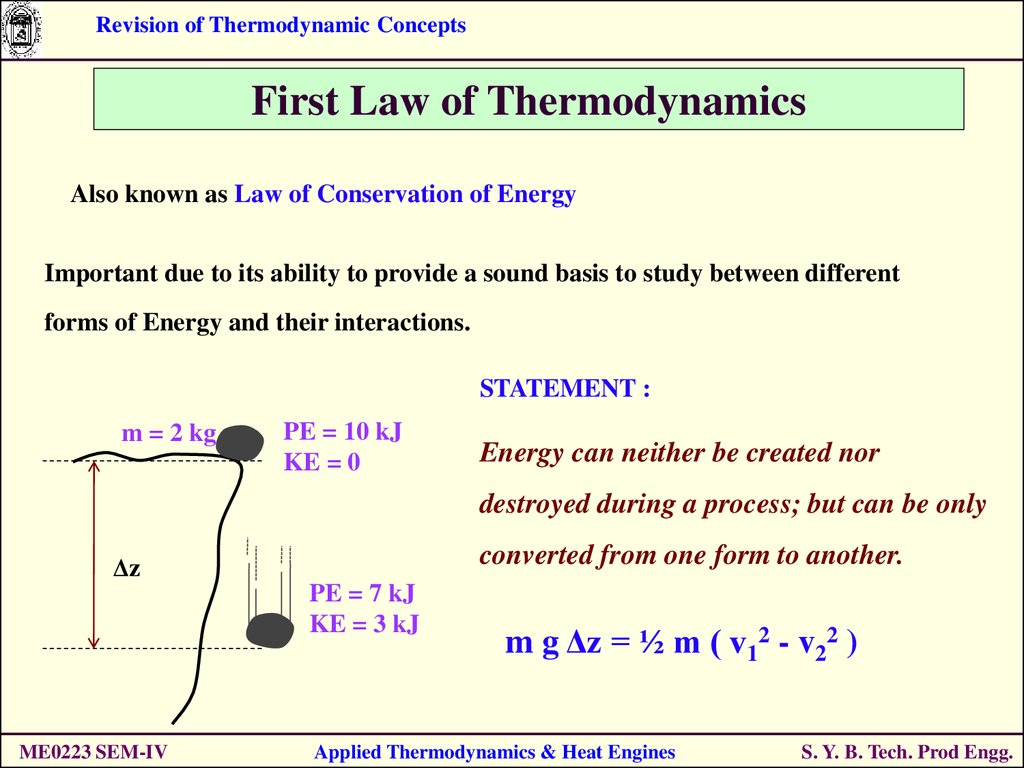
2) Energy can be converted from one form to another but not destroyed. Gibbs Equation Gsys Hsys TSsys Where, Gsys Gibbs energy change of the system Hsys enthalpy change of the system Ssys entropy change of the system T Temperature of the system This is known as the Gibbs equation. 1) Th total energy of an isolated system remains constant. Internal energy change is proportional to temperature variation ΔT and type of gas with the following equation: ΔU = Cv * n * ΔT, where Cv is molar heat capacity under constant volume. The First Law of Thermodynamics states that the energy lost from one part of the system evolves in another form, thus conserving total energy of the universe. It's quite tricky to estimate the precise value of internal energy, but it is possible to find thermal energy changes ΔU, which are described by the first law of thermodynamics: ΔU = Q - W, where Q denotes heat absorbed, and W is work done by gas.

Internal energy U is the sum of all kind of energies that are present in a system. Example: Cu + O2 + CO2 + H2O Cu2 (OH)2CO3 2) Select a Calculation Type.


 0 kommentar(er)
0 kommentar(er)
